(Taken from an email exchange after a recent holiday)
A huge problem we experienced while on a mountain holiday with a group of friends that caused an untold amount of heated debate, soul searching and hungry kids- cooking oeufs a la coque at altitude (boiled eggs for the unsophisticated).
We were in the mountains and had a terrible issue at breakfast. The Wife and kids decided to have boiled eggs to get a good start to the day. The mountain air was bound to make us hungry.
Usually the Wife keeps a very very careful eye on the time, 3½ minutes exactly, for her perfectly runny soft-boiled eggs.
But, when the eggs were pulled out of the pan, dipping soldiers at the ready, shell cracked open with a knife so the break is nice and clean, to our horror the egg was completely raw.
And now the eggs were open it is difficult to put them back in the boiling water to cook. It turns into some sort of poached-mess which you can’t dip the soldiers into. Not a good start to the day.
Somehow after this egg disaster the next day we tried boiled eggs again. But this time we added a bit of extra cooking time. ‘cos we ain’t dim
at 4 minutes we still had raw eggs.
The soldiers were getting restless.
Not to be deterred the next day we had another go. This time we only pulled one egg out at a time. After 5 minutes we still had an under-cooked egg.
At 5½ minutes the next egg was still under-cooked.
This was now getting serious, and number 2 was beginning to question the Wife’s egg boiling expertise. Which is very dodgy territory.
After 6 minutes the next egg was cracked open rather tentatively.
Bingo! A perfect soft-boiled egg. The rest were quickly served up to an eager gang at the breakfast table. The soldiers were a bit soggy but there were not too many complaints. After 3 days we had mastered the breakfast soft boiled egg challenge.
But, this saga started a discussion among the engineers in the group. And there are quite a few of them… Unfortunately, I couldn’t really help as I only work in powerpoint.
What was the calculation required to adjust the egg boiling time at altitude? And why? How did it go from 3½ minutes to 6 minutes? Almost doubling in cooking time. Obviously altitude/air pressure was a factor, but what was the maths/science behind it all?
To compound the issue the mountain hut did not have wifi to give us the solution! (This is a completely different issue that the kids are still pursuing with Childline).
Back at home a bit of internet searching (we have wifi at home) threw up a few very interesting articles on our subject.
A neat explanation and solution was found here:
http://newton.ex.ac.uk/teaching/CDHW/egg/
This is the summary:
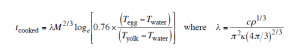
where ρ is density, c the specific heat capacity, and K thermal conductivity of ‘egg’. *
This looked like the solution to our boiled egg timing issue.
I took it upon myself to translate all of this into something more visual. I work in charts on powerpoint for time-challenged Execs…
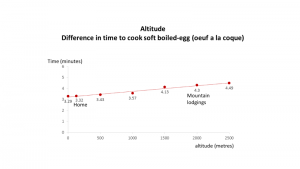
altitude does have an impact.
at room temperature (on the chart – the red line) cooking an egg at home (just above sea level) takes 3.32 minutes. but in the Mountain hut it will take 4½ minutes. due to a lower temperature to boil water (due to lower air pressure). from 100*c (at home) to approx 98*c (in mountain hut). if the temperature of the water is lower it will take longer to cook. this 3.32 minutes is very close to the actual time the Wife cooks the egg. science looks like it supports the Wife.
Altitude adds about 1 minute to the time to soft boil an egg.
But this did not explain the much longer time we took to boil our eggs.
One factor the Wife didn’t take into account while cooking was the friends were in the habit of keeping the eggs in the fridge. and she keeps them in a cupboard next to the fridge.
Perhaps equally interesting is the effect of keeping the eggs in the fridge or not. This obviously affects the cooking time. This is quite a reasonable assumption as the cooler something is the longer it will take to cook.
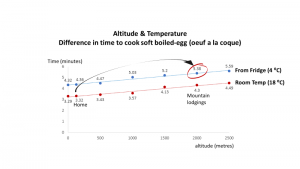
This is shown by a jump from the red line to the blue line. thus for home it goes from 3.32 minutes to 4.36 minutes at the same altitude. a minute longer to soft boil an egg from the fridge rather than outside the fridge.
So at 2000 metres altitude, when we take an egg from the fridge and want a perfect soft boiled egg, it takes 5.38 minutes.
Thus when we combine the altitude and fridge effect together we get an extra 2 minutes to boil an egg from our original experience. (the black arrow)
While these are theoretical calculations, we saw 2½ minutes extra, the general science supports what we experienced.
The engineers in the gang certainly found this very interesting.
Although the wife commented, “You’re such a busy man !! don’t you have any real work to do?”
note* This solution does contain some approximations as some commentators have noted. For a more accurate treatment, one would need to account for the thermal properties of the white, yolk and shell are all different. The egg would need to be treated as three concentric, ellipsoids with Dirichlet boundary-conditions at the water-shell interface and Neuman boundary-conditions for the shell-white and white-yolk parts. Changes in its thermal properties when the white changes state from liquid to gel, and the latent heat associated with this change would also need to be accounted for.
Great to see a new post!
And also good to see your work in developing ‘highly sophisticated econometric (type) models’ continues. Keep up the good work.